Tetanus immune globulin (TIG) is recommended as treatment for persons with tetanus infection. TIG can help to remove unbound tetanus toxin but cannot affect toxin already bound to nerve endings.
Intravenous immune globulin (IVIG) contains tetanus antitoxin and may be used if TIG is not available.
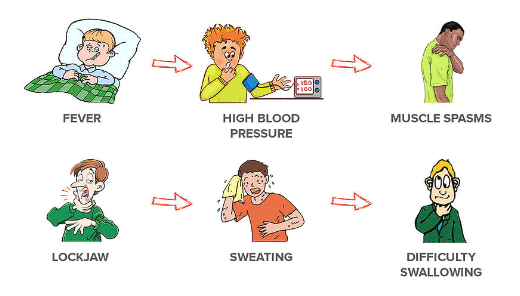
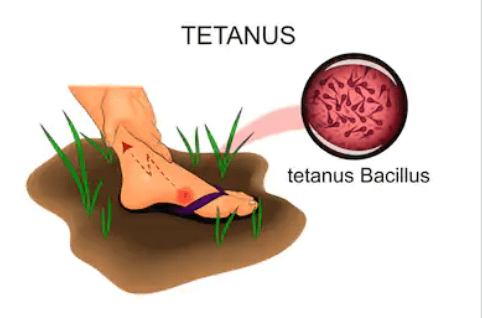
Medical management of tetanus should prioritize wound cleaning, through removal of all foreign material and necrotic tissue. If the patient is experiencing tetanic spasms, maintenance of an adequate airway is critical.
Immunization with tetanus toxoid should occur only after the person’s condition is stable.
Routine Wound Management Guide for Tetanus Prophylaxis with TIG (CDC Pink Book – Tetanus)
|
History of absorbed tetanus toxoid-containing vaccines |
| Wound Classification (Treatment) |
Unknown or <3 doses |
>=3 doses |
| Clean, minor wounds (DTap, Tdap or Td) |
Yes |
No* |
| All other wounds (DTaP, Tdap, Td) |
Yes |
No** |
| Clean, minor wounds (TIG) |
No |
No |
| All other wounds (TIG)*** |
Yes |
No |
*Yes, if ≥10 years since the last tetanus toxoid-containing vaccine dose.
**Yes, if ≥ 5 years since the last tetanus toxoid-containing vaccine dose.
***People with severe immunodeficiency or HIV infection, who have contaminated wounds, should also receive TIG, regardless of tetanus immunization history.