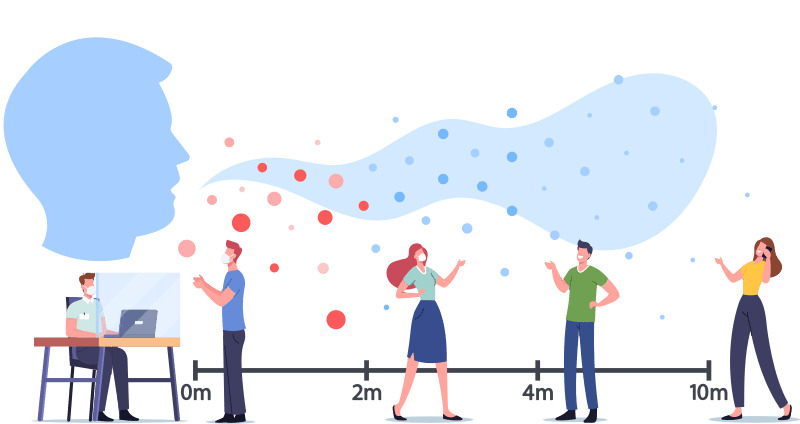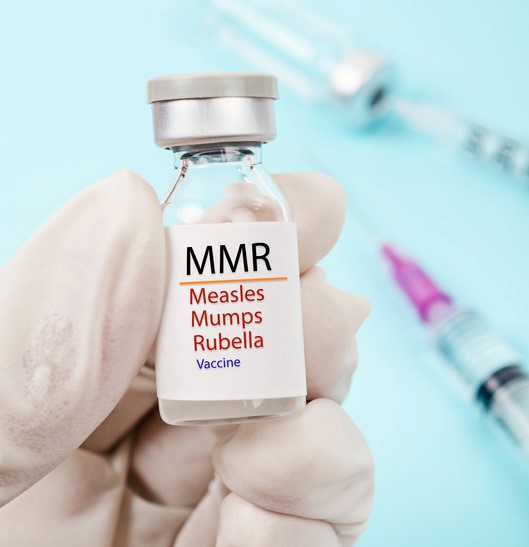
Rubella can be prevented with MMR or MMRV vaccines. California requires children enrolling in school or childcare receive at least one dose of rubella-containing vaccine.
The CDC recommends that children receive two doses of MMR
or MMRV:
- First dose at 12 – 15 months of age, and their second dose at 4 – 6 years of age (prior to starting kindergarten).
- Infants 6 – 11 months of age should get one dose of a rubella-containing vaccine prior to traveling internationally.
- Other vaccines can be given at the same time as the MMR or MMRV vaccine.
Adults who are unvaccinated or are unsure of their vaccination status should get at least one dose of MMR vaccine, as 1 dose is sufficient for most adults.
Certain adults may need 2 doses. Adults who are going to be in a setting that poses a high risk for measles or mumps transmission should make sure they have had two doses separated by at least 28 days. These include:
- Students at post-high school education institutions
- Healthcare personnel
- International travelers
Women of childbearing age who are planning to become pregnant should check with their doctor to ensure they’re vaccinated before getting pregnant. They should avoid getting pregnant for at least 4 weeks after receiving the MMR vaccine.
If you are unsure about your vaccine status, review your vaccine records and talk with your doctor about getting vaccinated.